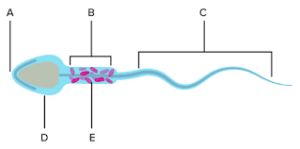
Mature spermatozoa, with their characteristic head, midpiece, and long tail, play a fundamental role in male fertility. These sperm cells are the product of a complex process called spermatogenesis, occurring in the testicles. During this transformation, round germ cells develop into elongated sperm cells, a metamorphosis that relies on the precise orchestration of specialized structural proteins.
One such protein, ACTL7B, appears to be pivotal in this intricate dance. Researchers from the University Hospital Bonn (UKB) and the Transdisciplinary Research Unit “Life & Health” at the University of Bonn have unveiled the significant role of ACTL7B. Their groundbreaking study, published in the journal Development, sheds light on how the loss of this structural protein hinders spermatogenesis and leads to male infertility.
The Backbone of Spermatogenesis
Spermatogenesis, the continuous production of sperm cells, is essential for male fertility. This process entails the remarkable transformation of round germ cells into elongated sperm cells, demanding the precise reorganization of specialized structural proteins. ACTL7B is one such protein, known for being exclusively produced during the maturation of male sperm in humans and mice. This exclusivity suggests its paramount importance during this developmental phase. Prof. Hubert Schorle, the corresponding author of the study and an expert from the Institute of Pathology at UKB, as well as a member of the Transdisciplinary Research Area “Life & Health” at the University of Bonn, emphasizes the significance of ACTL7B in spermatogenesis.
Understanding ACTL7B’s Role
To delve deeper into the role of ACTL7B in spermiogenesis, Prof. Schorle’s team employed gene-editing technology to create a mouse model with a mutation in the Actl7b gene. This mutation resulted in the complete loss of function of ACTL7B. As first author Gina Esther Merges, a doctoral student in Professor Schorle’s laboratory, explains,
Without ACTL7B, development is blocked, the cells often remain in a roundish shape, usually do not form the elongated, typical sperm shape, and die to a large extent.”
In their research, the scientists from Bonn discovered that ACTL7B plays a vital role in the reorganization of the cytoskeleton of spermatids. They conducted mass spectrometric analyses, which revealed two interaction partners of ACTL7B, DYNLL1 and DYNLL2. Prof. Schorle clarifies, “We were able to show that without the structural protein, DYNLL1 and 2 are not correctly localized in the round spermatids. Since it is probably a larger protein complex with further interaction partners, we attribute the above-described effect to a loss of temporally and spatially precisely regulated and targeted redistribution of these proteins.”
The study underscores why sperm from male mice with a mutated Actl7b gene cannot attain the characteristic shape, rendering the animals infertile. Furthermore, this research aligns with other studies suggesting that levels of the ACTL7B protein may be reduced in certain cases of male infertility. “Our study illuminates that mutations in the Actl7b gene could underlie male infertility,” notes Prof. Schorle. This groundbreaking insight not only expands our understanding of spermatogenesis but may also hold the key to addressing male infertility issues in the future.